
What You Should Know:
– RapidAI, a pioneer in deep clinical AI, announced U.S. FDA clearance for five new imaging modules—Rapid DeltaFuse™, Rapid LMVO, Rapid MLS, Rapid OH, and Rapid Aortic for measurement.
– The expansion reinforces the Rapid Enterprise™ Platform’s focus on bringing deep clinical intelligence and seamless workflow integration across the entire patient journey.
Deep Clinical AI: Shifting Beyond Triage to Full Patient Management
RapidAI’s latest FDA clearances underscore its commitment to building deep clinical AI—algorithms that move beyond simple triage to support superior decision-making in both acute and long-term settings.
The five new modules focus on providing precision, quantification, and automation across neurology and vascular care:
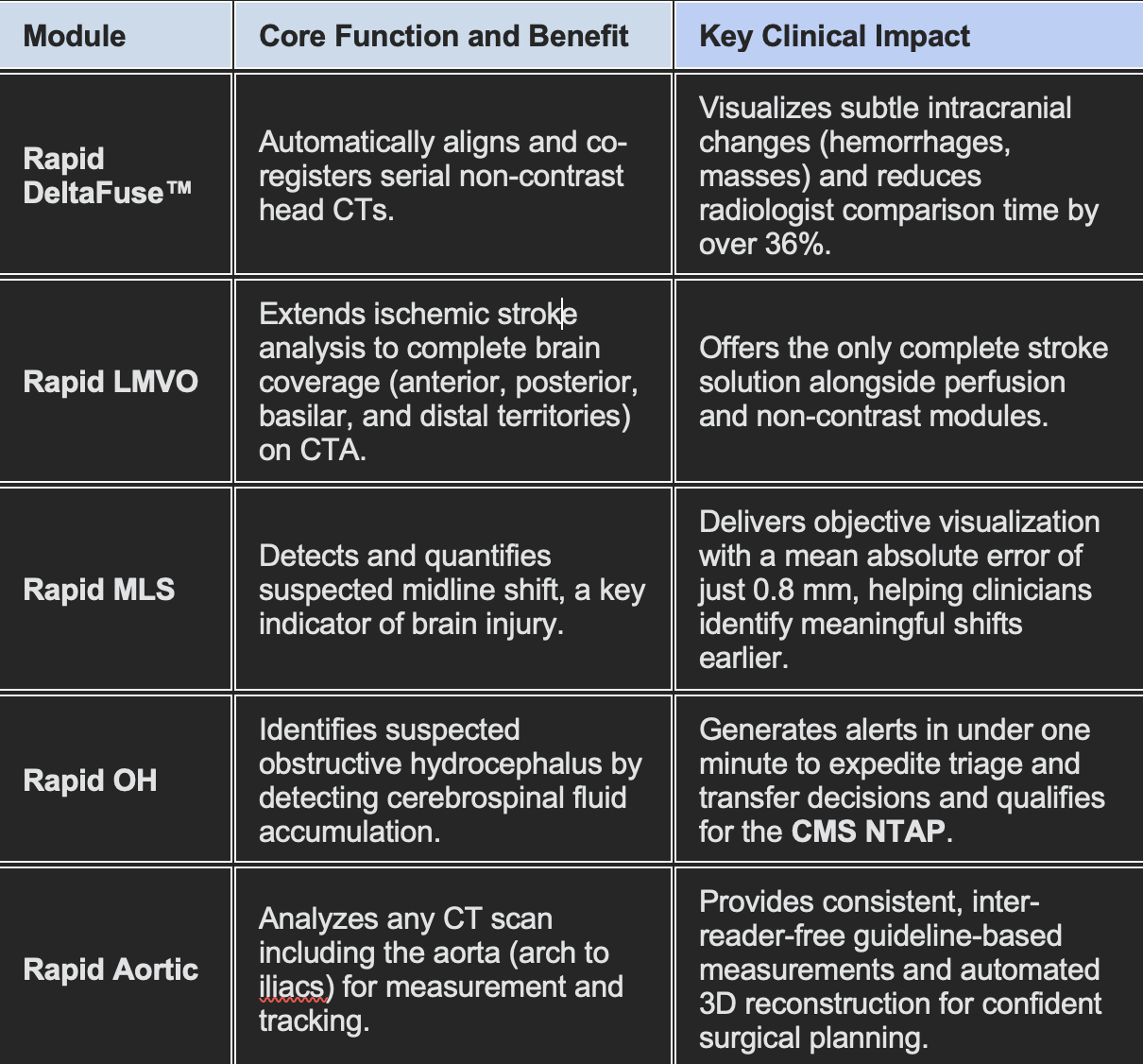
The new capabilities directly address cognitive burden for radiologists. By pairing imaging precision with automation, the modules reduce interpretation time and eliminate manual measurement variability.

